Agitation
2. the intentional, usually mild, disturbance of the skin, mucosa, or other surface (e.g., with a wooden interdental cleaner or probe instrument) to determine if infection or disease is present. If agitated surfaces bruise or bleed easily, or are otherwise disrupted (e.g., develop a lesion), the presence of a pathologic condition should be suspected.
3. a psychosomatic condition represented by uncontrollable or excessive body movements. The psychologic aspect may often indicate the presence of unresolved stress.
Effects of agitation and aeration on Production of Hexokinase
Effects of agitation and aeration on growth
Sterile Filtration
Clear liquids that would be damaged by heat, irradiation or chemical sterilization can be sterilized by mechanical filtration. This method is commonly used for sensitive pharmaceuticals and protein solutions in biological research. A filter with pore size 0.2 µm will effectively remove bacteria. If viruses must also be removed, a much smaller pore size around 20 nm is needed. Solutions filter slowly through membranes with smaller pore diameters. Prions are not removed by filtration. The filtration equipment and the filters themselves may be purchased as presterilized disposable units in sealed packaging, or must be sterilized by the user, generally by autoclaving at a temperature that does not damage the fragile filter membranes. To ensure sterility, the filtration system must be tested to ensure that the membranes have not been punctured prior to or during use.
To ensure the best results, pharmaceutical sterile filtration is performed in a room with highly filtered air (HEPA filtration) or in a laminar flow cabinet or "flowbox", a device which produces a laminar stream of HEPA filtered air.
Radiation Sterilization
Methods exist to sterilize using radiation such as X-rays, gamma rays, or subatomic particles.
- Gamma rays are very penetrating and are commonly used for sterilization of disposable medical equipment, such as syringes, needles, cannulas and IV sets. Gamma radiation requires bulky shielding for the safety of the operators; they also require storage of a radioisotope (usually Cobalt-60), which continuously emits gamma rays (it cannot be turned off, and therefore always presents a hazard in the area of the facility).
- X-rays are less penetrating than gamma rays and tend to require longer exposure times, but require less shielding, and are generated by an X-ray machine that can be turned off for servicing and when not in use.
- Ultraviolet light irradiation (UV, from a germicidal lamp) is useful only for sterilization of surfaces and some transparent objects. Many objects that are transparent to visible light absorb UV. UV irradiation is routinely used to sterilize the interiors of biological safety cabinets between uses, but is ineffective in shaded areas, including areas under dirt (which may become polymerized after prolonged irradiation, so that it is very difficult to remove). It also damages many plastics, such as polystyrene foam.
- Further information: Ultraviolet Germicidal Irradiation
- Subatomic particles may be more or less penetrating, and may be generated by a radioisotope or a device, depending upon the type of particle.
Irradiation with X-rays or gamma rays does not make materials radioactive. Irradiation with particles may make materials radioactive, depending upon the type of particles and their energy, and the type of target material: neutrons and very high-energy particles can make materials radioactive, but have good penetration, whereas lower energy particles (other than neutrons) cannot make materials radioactive, but have poorer penetration.
Irradiation is used by the United States Postal Service to sterilize mail in the Washington, DC area. Some foods (e.g. spices, ground meats) are irradiated for sterilization .
Chemical Sterilization
Chemicals are also used for sterilization. Although heating provides the most reliable way to rid objects of all transmissible agents, it is not always appropriate, because it will damage heat-sensitive materials such as biological materials, fiber optics, electronics, and many plastics.
Ethylene oxide (EO or EtO) gas is commonly used to sterilize objects sensitive to temperatures greater than 60 °C such as plastics, optics and electrics. Ethylene oxide treatment is generally carried out between 30 °C and 60 °C with relative humidity above 30% and a gas concentration between 200 and 800 mg/L for at least three hours. Ethylene oxide penetrates well, moving through paper, cloth, and some plastic films and is highly effective. Ethylene oxide sterilizers are used to process sensitive instruments which cannot be adequately sterilized by other methods. EtO can kill all known viruses, bacteria and fungi, including bacterial spores and is satisfactory for most medical materials, even with repeated use. However it is highly flammable, and requires a longer time to sterilize than any heat treatment. The process also requires a period of post-sterilization aeration to remove toxic residues. Ethylene oxide is the most common sterilization method, used for over 70% of total sterilizations, and for 50% of all disposable medical devices.
The two most important ethylene oxide sterilization methods are: (1) the gas chamber method and (2) the micro-dose method. To benefit from economies of scale, EtO has traditionally been delivered by flooding a large chamber with a combination of EtO and other gases used as dilutants (usually CFCs or carbon dioxide ). This method has drawbacks inherent to the use of large amounts of sterilant being released into a large space, including air contamination produced by CFCs and/or large amounts of EtO residuals, flammability and storage issues calling for special handling and storage, operator exposure risk and training costs. Because of these problems a micro-dose sterilization method was developed in the late 1950s, using a specially designed bag to eliminate the need to flood a larger chamber with EtO. This method is also known as gas diffusion sterilization, or bag sterilization. This method minimize the use of gas.[3]
Bacillus subtilis, a very resistant organism, is used as a rapid biological indicator for EO sterilizers. If sterilization fails, incubation at 37 °C causes a fluorescent change within four hours, which is read by an auto-reader. After 96 hours, a visible color change occurs. Fluorescence is emitted if a particular (EO resistant) enzyme is present, which means that spores are still active. The color change indicates a pH shift due to bacterial metabolism. The rapid results mean that the objects treated can be quarantined until the test results are available.
Ozone is used in industrial settings to sterilize water and air, as well as a disinfectant for surfaces. It has the benefit of being able to oxidize most organic matter. On the other hand, it is a toxic and unstable gas that must be produced on-site, so it is not practical to use in many settings.
Chlorine bleach is another accepted liquid sterilizing agent. Household bleach consists of 5.25% sodium hypochlorite. It is usually diluted to 1/10 immediately before use; however to kill Mycobacterium tuberculosis it should be diluted only 1/5. The dilution factor must take into account the volume of any liquid waste that it is being used to sterilize.[4] Bleach will kill many organisms immediately, but for full sterilization it should be allowed to react for 20 minutes. Bleach will kill many, but not all spores. It is highly corrosive and may corrode even stainless steel surgical instruments.
Glutaraldehyde and formaldehyde solutions (also used as fixatives) are accepted liquid sterilizing agents, provided that the immersion time is sufficiently long. To kill all spores in a clear liquid can take up to 12 hours with glutaraldehyde and even longer with formaldehyde. The presence of solid particles may lengthen the required period or render the treatment ineffective. Sterilization of blocks of tissue can take much longer, due to the time required for the fixative to penetrate. Glutaraldehyde and formaldehyde are volatile, and toxic by both skin contact and inhalation. Glutaraldehyde has a short shelf life (<2>
Ortho-phthalaldehyde (OPA) is a chemical sterilizing agent that received Food and Drug Administration (FDA) clearance in late 1999. Typically used in a 0.55% solution, OPA shows better myco-bactericidal activity than glutaraldehyde. It also is effective against glutaraldehyde-resistant spores. OPA has superior stability, is less volatile, and does not irritate skin or eyes, and it acts more quickly than glutaraldehyde. On the other hand, it is more expensive, and will stain proteins (including skin) gray in color.
Hydrogen peroxide is another chemical sterilizing agent. It is relatively non-toxic once diluted to low concentrations (although a dangerous oxidizer at high concentrations), and leaves no residue.
Low Temperature Plasma sterilization chambers use hydrogen peroxide vapor to sterilize heat-sensitive equipment such as rigid endoscopes. A recent model can sterilize most hospital loads in as little as 20 minutes. The Sterrad has limitations with processing certain materials such as paper/linens and long thin lumens. Paper products cannot be sterilized in the Sterrad system because of a process called cellulostics, in which the hydrogen peroxide would be completely absorbed by the paper product.
Hydrogen peroxide and formic acid are mixed as needed in the Endoclens device for sterilization of endoscopes. This device has two independent asynchronous bays, and cleans (in warm detergent with pulsed air), sterilizes and dries endoscopes automatically in 30 minutes. Studies with synthetic soil with bacterial spores showed the effectiveness of this device.
Dry sterilization process (DSP) uses hydrogen peroxide at a concentration of 30-35% under low pressure conditions. This process achieves bacterial reduction of 10-6...10-8. The complete process cycle time is just 6 seconds, and the surface temperature is increased only 10-15 °C (18 to 27 °F). Originally designed for the sterilization of plastic bottles in the beverage industry, because of the high germ reduction and the slight temperature increase the dry sterilization process is also useful for medical and pharmaceutical applications.
Peracetic acid (0.2%) is used to sterilize instruments in the Steris system.
Prions are highly resistant to chemical sterilization. Treatment with aldehydes (e.g., formaldehyde) have actually been shown to increase prion resistance. Hydrogen peroxide (3%) for one hour was shown to be ineffective, providing less than 3 logs (10-3) reduction in contamination. Iodine, formaldehyde, glutaraldehyde and peracetic acid also fail this test (one hour treatment). Only chlorine, a phenolic compound, guanidinium thiocyanate, and sodium hydroxide (NaOH) reduce prion levels by more than 4 logs. Chlorine and NaOH are the most consistent agents for prions. Chlorine is too corrosive to use on certain objects. Sodium hydroxide has had many studies showing its effectiveness.
Sterilization
Sterilization (or sterilisation) refers to any process that effectively kills or eliminates transmissible agents (such as fungi, bacteria, viruses, prions and spore forms etc.) from a surface, equipment, foods, medications, or biological culture medium. Sterilization can be achieved through application of heat, chemicals, irradiation, high pressure or filtration.
There are two types of sterilizations. That is
- a)physical sterilization
- b)chemical sterilization
Motion on Therapeutic uses of cloning
Some exaggerated claims have recently been made for alternative sources of stem cells, for example from human adult cells or placental material. While recent advances in these areas are indeed encouraging, scientists are urging great caution over assuming universal therapeutic success with any one method, when these are still very early stages in research. One speculative means to produce stem cells may already be rejected on ethical grounds, however. This is the production of non-viable human embryos within cow's eggs. The idea would be to take a human cell and perform a nuclear transfer into a denucleated cow's egg. Passing an electric current would fuse the two and stimulate the human cell to divide as though it were a human embryo, but one which was not viable. At the blastocyst stage of division, the stem cells would be removed and cultured as human somatic cells. This raises many serious uncertainties and risks, not least whether the use of a cow's egg as a host for the human cell had no adverse effect on the eventual human cell lines. It would raise immense ethical problems. Even though it would avoid the creation of a human embryo, the mixing of human and animal genetic material at such a profound level would raise a major intrinsic ethical objection for many people.
Cloning for Therapeutic Purposes
The vote in Parliament extended the uses of embryos to include making embryonic stem cells for serious human disease. Most of cells needed would be taken from existing "spare" embryos from IVF treatments. The careless use of the word cloning to describe this caused much confusion, but it does point to an issue of concern. MP's did not have any chance to vote on the cloning of embryos, because it was technically legal due to a loophole in the Act. The present Human Fertilisation and Embryology Act (1990) allows the creation of embryos for limited research purposes, mainly to do with infertility. On these grounds, the creation of cloned embryos has been forbidden, because this would be seen as reproductive cloning. By creating a new legal use for creating embryos for a non-reproductive use - to create stem cells - the Commons vote automatically allowed the cloning of embryos for this purpose, without ever voting on it. The influential European Commission ethical advisory panel reported on these issues in November 2000 and drew an ethical line at cloning embryos, as did a vote in the European Parliament. The UK vote should not be seen as a mandate to allow cloned embryos also, because this has not been put to a proper democratic test. We need early primary legislation on therapeutic as well as reproductive uses of cloning.
Cloned human embryos present several ethical problems. Firstly, it seems illogical to allow the creation of a cloned human embryo knowing full well one would have to destroy it on ethical grounds, because it was unethical to allow it to go to term to produce a cloned baby. The second objection is that this involves the deliberate creation of an embryo for other than reproductive purposes, although this is not specific to cloning. The use of "spare" embryos from fertility treatments would be a use of an embryo that would be destroyed anyway.
Thirdly, there is a gradualism argument. Once cloned human embryos were created, it would be much easier for someone misguided enough to go the next step and allow them to be implanted, or for someone rich enough to seek a clandestine "off-shore" treatment. This underlines the need for clear national laws, in those states which do not currently have them, to outlaw the practice of human cloning worldwide.
The creation and use of cloned human embryos should not be allowed as a general therapeutic procedure. We urge, however, that a priority should be put on nuclear transfer research which aims at avoiding use of embryos, by direct programming from one adult body tissue type to another. One could take, perhaps, a blood sample and reprogramme directly into becoming, say, a set of nerve cells. This is of course even more speculative than the methods discussed above, but several routes have recently been suggested. Ethically this would remove most of the above objections.
There is also a further reason. The ethics committee of Roslin's collaborators, the Geron Biomed company, has urged that the technique should have the widest applicability and not be simply a treatment for the rich. It is very unlikely that enough human donor eggs could ever be provided to treat the millions of potential patients across Europe. It would therefore probably be essential to find a method of producing replacement cells without using embryos. On present evidence, however, this would probably be impossible without some human embryo research to work out the method. This poses a deep ethical dilemma whether a very limited and fixed number of experiments should be allowed to obtain the data necessary to avoid any such use of embryos in future. Some would reluctantly argue for very limited research for this sole purpose, but if it seemed unlikely to succeed, then it should stop, and not proceed to use embryos routinely for cell therapies.
Cloning and Stem cells; the animal crossover
In 1997, SRT reported to the General Assembly on human and animal cloning. At that point, and since, cloning research was almost entirely confined to animals. The human interest in cloning was in the possibilities that reproductive human cloning might be attempted, despite the declared intention of the UK government never to allow it, and the unlikelihood that this would ever be safe. The persistent question in the media is that somewhere, someone will attempt it in the USA where private sector research is essentially unregulated, or in an "offshore" situation outside any restrictive jurisdiction. It began in animals to help in genetically engineering sheep more effectively to produce pharmaceuticals in their milk, but for some sections of the media, it is still all about who would clone the first human baby, however dangerous and unethical this would be. Motion on Reproductive Cloning Legislation
In 1998, the isolation of human embryonic stem cells was announced in the USA, extending from many years of work in mouse stem cells. These are special cells in the early embryo before it begins to differentiate. At this point, they can turn into any type of cell in the human body. Two years ago, US scientists found a way to isolate them. Using special chemical treatments, they believe they can direct them into becoming any type of human cell they choose - skin, heart muscle, nerve cells, etc. This opens up a possibility to create replacement cells to inject into patients suffering from a wide range of diseases which cause irreversible cell degeneration, like Parkinson's, some heart conditions and diabetes. In December 2000, the UK Parliament gave its approval to research using these techniques to produce replacement cells for a range of human diseases where cell degeneration is crucial. Thus far, most of the research is however done in animals, for example into the ways in which early embryonic cells go through the strange process of differentiation. Mice have been genetically engineered to induce a form of Parkinson's disease and tests have been done on replacement cells as a possible mimic of a human therapeutic technique. Research in human stem cells will now proceed, but many of the discoveries may also feed back into other mammalian stem cells, and the spiral of development will continuos.
GM Animals as Mdels of Human Desease
In "Engineering Genesis" we pointed the anomaly of the vast increase in model mice against the general European trend in animal research of the "3 R's" - reduce, refine and replace. We suggested that the use had become too automatic, and steps needed to be taken to make researchers think twice before using mice.14 The fear is that mice have ceased to be seen as animals at all, in this context, and are merely items in a research catalogue.
GM Pigs for Xenotransplantation
The first is the rapid rejection by the human immune system of organs from another species. Pigs have to be genetically modified to try to overcome this. Several human genes have to be added to the pig to send "human" signals that would prevent the human immune system not to reject the organ. There are as many as four genes involved. This requires multiple gene changes, something which has never been done before in a large animal, and is hard to achieve even in plants. It also requires knocking out genes in the pig which would trigger the rejection. So far most genetic engineering has only added genes. The nuclear transfer cloning of piglets by PPL in 2000 has opened a potential way to do this, if gene deletion were done in vitro in cells, and if pigs could be "grown" from these genetically altered cells. This is uncharted scientific territory. No one knows if this can be done to overcome rejection to a sufficient degree for a viable medical procedure.
The second technical barrier is the remote risk of the transfer of a pig retrovirus to humans, to which humans might not be immune. The concern is less for the patient, who is probably terminally ill anyway, but about the possibility that such a virus might be transmitted to the family and then out into the wider human population. This is an extremely remote risk, in terms of probability, but it could have epidemic consequences were such a chain of events to occur. The origins of HIV and the trans-species aspect of BSE both present scenarios sufficient for the government to have a moratorium on clinical trials. Its advisory body on xenotransplantation has recommended draconian restrictions on the patient and family, were clinical trials ever to begin. The implications and evaluation of this lie beyond the scope of the present report, but it clearly indicates the delicacy and complexity of the animal - human interface.
For the present report our main ethical concern is to review the use of animals in this way. To breed and genetically engineer an animal solely to remove an entire live organ represents a different use of animals from anything humans have done before. It is a large leap from using pig heart valves, which are merely dead tissue with convenient elastic properties. The "yuk reaction", which the idea of xenotransplantation often prompts, suggests that having a complete animal heart inside oneself poses underlying questions beyond mere unfamiliarity. Some respond by contending that if we accept eating pigs, it is even more justified to use them this way to save human life. This purely consequential way of framing the issue is shallow, however. Logically it would justify doing literally anything to a pig in order to save human life. It is at odds with any ethical perspective based on the notion that animals have intrinsic value, and the implication from the biblical examples that animal use eventually has limits. The landmark Banner Report on animal ethics established that there are some things we should never do to animals, no matter what the reason.13 This "ham sandwich argument" also side steps other issues. Unlike eating animals, there is no parallel to xenotransplantation in nature. The fact that xenotransplantation is unnatural, in that sense, may not necessarily make it wrong, but it prompts a question whether this is an acceptable extension of human use of animals from traditional suppliers of food, clothing, traction, transport and manure?
Pharmaceuticals on GM Animals
The 1997 Assembly report on cloning acknowledged that this did not raise undue ethical problems. The use of sheep milk is traditional and therefore to produce a particular protein in the milk would not seem an undue departure from the current situation, particularly since the sheep version of AAT is produced by the animal, albeit in the liver rather than in milk. The intervention in the animal is judged to be small, the human medical need being addressed is considerable, and other routes to the protein are much more difficult. Indeed, it could be argued as a genuine partnership, in which humans give especial husbandry and care of the sheep in exchange for a valuable product in the sheep's milk. No welfare concerns have arisen from this particular example.In general this is an area where we would say "Yes, provided." One such proviso arose in research to produce a more active protein erythropoietin showed unacceptable welfare effects for the animals, which led to the trials rightly being terminated.
GM Animals
Is genetic engineering inherently wrong, irrespective of its application or its consequences?
Some Christians may consider that to change a single gene in an animal would be attempting to change God's best design, upsetting the wisdom inherent in the natural order by humans who did not know the full extent of the unprecedented changes they were making. Some would say we should not genetically engineer animals in any way we would not do in humans. The SRT report to the 1999 Assembly on genetically modified food and crops established grounds that manipulating genes and transferring them amongst widely varying species did not in itself violate a fundamental limit in the nature of things. The same would apply to animals, but, as with GM crops, there are important caveats. The nature of an animal, like plants and humans, is more than the mere sum of its genes but lies in the wider essence of the creature as a whole. Animals are also in constant genetic variation. To change one or two genes is not like changing a fixed blueprint, which would irretrievably violate the animal, unless the result brought about a severe impairment or suffering to the animal. We must therefore ask whether a particular genetic change poses special problems in relation to the nature of the animal, and also what regard we give to different types of animals, for example, primates, pigs, mice, frogs and midges.
A "No, unless" approach might allow uses where the prime benefit was to the animal, such as increased disease resistance, or in cases where a major human benefit could be achieved with minimal interference in the animal. It would be more critical about increased growth rate in animal production, whether the level or nature of intervention was permissible, and what motives were driving it. It would ask if there were better ways to the same end without manipulating the animals.
Theological Reflection on the human use of animals
The relationship of humans to God's creation has been expressed most often in Calvin's notion of the steward. God gives humans a special duty both to develop the natural world - and hence the use of technology - but also to take care of it - which puts limits on our activities. Stewardship means that humankind is answerable not merely to future human generations, but to God, the divine owner, for how we have looked after his estate. Alongside this Ruth Page introduced the notion of companionship, to reflect that we are also fellow creatures in a shared creation.Thus while God puts animals under human subjugation for a wide variety of uses, they are still God's creatures first, and humans will have to give an account to God for their care of them. Old Testament injunctions such as "Do not muzzle an ox when it is treading out the grain", "Do not boil a kid goat in its mother's milk." (Deuteronomy 25:4 and 14:21) imply that wider principles of relationship set restraints on human uses.
This contrasts with historical views of animals as merely there for human purposes. or the view that they are not radically different from us scientifically or morally. Aspects and characteristics which human and animal hold in common, like both being creatures, being "subjects of a life" or being sentient, do not mean that humans cannot eat animals or use them for traction and carriage. The notion of animal "rights" is criticised because in a Christian understanding there are no rights without corresponding responsibilities, and animals do not have responsibilities towards humans it is meaningless to give them rights.Rather we would stress our duties towards them under God.
Commercial animal production by selective breeding would be allowed, but not to every degree possible. Limits are exceeded when this is taken as an end in itself, or if it becomes so dominated by a functional view of the animal under pressures of economic efficiency that wider principles of God's creation are overridden. The case of poultry production has shown that when taken to such degrees that harms, distortions, disablement or impairment of function begin to emerge, a good end would have been taken too far.
Introduction og Genetic Engineering
While the controversy of GM foods has been so much in the news, the genetic engineering of animals has been comparatively ignored. It was one of the main themes of the SRT Project study "Engineering Genesis",1 in which context it was mentioned briefly in SRT's 1998 Assembly report as well as in the 1997 National Mission report on Animal and Human Cloning.2 The recent genetic engineering of a monkey in the USA has now brought to the fore some important issues about the research on animals for human benefits. The dramatic developments in cloning and embryonic human stem cells are raising another basic question of the increasingly blurred borderline between animal and human research. Research done on animals today, like cloned sheep and mouse stem cells, can rapidly become applied for use in humans. Insights from human examples feed back into animal research. In this report, we wish to examine how far we may use and modify animals for human uses, and the relationship between biotechnology in animals and in humans. By way of example, we discuss the latest developments in xenotransplantation, animal models of human disease, and cloning and stem cell technology.
The genetic engineering of animals has stimulated much public discussion, and raises a number of important questions about human intervention in animals. Despite much research, it has not found significant application in animal production for meat, milk, eggs, wool or hides. So far it seems to offer few advantages over conventional breeding and the promising field of genetic marker assisted selection. Genetic engineering in animals has primarily been in novel applications in medicine, and in particular making pharmaceuticals in the milk of farm animals, pig organs in humans, and use of mice and other animals as models of human disease.
Genetic Engineering
Kinetic Properties
The Michaelis constant has been found to decrease by more than one order of magnitude when substrate of opposite charge of that of the carrier matrix was used. Again, this only happened at low ionic strengths, and when neutral substrates were used. The electrostatic potential was calculated by insertion of the Maxwell-Boltzmann distribution into the Michaelis-Menten equation using the changes in Michaelis constant. Good agreement was obtained with the value for the electrostatic potential calculated from the pH-activity shifts.
It is recognized that the kinetic constants measured with immobilized enzymes are not true kinetic constants equivalent to those obtained in homogeneous reactions. They are apparent values because of the effects of diffusion and partitioning. Hence, maximum velocity and Michaelis constants should be referred to as apparent Vmax and apparent Km.
The diffusion of substrate from the bulk solution to the micro-environment of an immobilized enzyme can limit the rate of the enzyme reaction. The rate at which substrate passes over the insoluble particle affects the thickness of the diffusion film, which in turn determines the concentration of substrate in the vicinity of the enzyme and hence the rate of reaction.
Molecular weight of the substrate can also play a large role. Diffusion of large molecules will obviously be limited by steric interactions with the matrix. This is reflected in the fact that the relative activity of bound enzymes towards high molecular weight substrates has generally been found to be lower than towards low molecular weight substrates. This, however, may be an advantage in some cases, since the immobilized enzymes may be protected from attack by large inhibitor molecules.
Enzyme Activity
- Spacing (steric hindrance)
- pH
- Temperature
- Substrate Concentration (Michaelis-Menten Kinetics)
Benefits of Immobilizing an Enzyme
Benefits of Immobilizing an Enzyme
There are a number of advantages to attaching enzymes to a solid support and a few of the major reasons are listed below:
- Multiple or repetitive use of a single batch of enzymes
- The ability to stop the reaction rapidly by removing the enzyme from the reaction solution (or vice versa)
- Enzymes are usually stabilized by bounding
- Product is not contaminated with the enzyme (especially useful in the food and pharmaceutical industries)
- Analytical purposes - long 1/2-life, predictable decay rates, elimination of reagent preparation, etc.
Methods of Immobilization
The surface on which the enzyme is immobilized is responsible for retaining the structure in the enzyme through hydrogen bonding or the formation of electron transition complexes. These links will prevent vibration of the enzyme and thus increase thermal stability. The micro environment of surface and enzyme has a charged nature that can cause a shift in the optimum pH of the enzyme of up to 2 pH units. This may be accompanied by a general broadening of the pH region in which the enzyme can work effectively, allowing enzymes that normally do not have similar pH regions to work together.
Immobilized Enzyme
Standardization of Human Diploid cell cultivation
Cell cultivation
Studies investigating a specific sub-clone and cultivation parameters can easily become an extensive task. DASGIP offers a parallel small-scale cultivation solution providing tightly controlled conditions that integrate separate modules for monitoring, dosing, feeding and documentation
 Samples can be taken from one of up to 18 ports of the reactors' sterile head plates.
Samples can be taken from one of up to 18 ports of the reactors' sterile head plates. Individual parameters of a single or full culture can be isolated and optimized.
Individual parameters of a single or full culture can be isolated and optimized.The DASGIP system was utilised in a study into the impact of a small-scale, stirred cultivation system with oxygen and pH control, using a CHO cell line secreting the recombinant protein (MUC1-Fc). The system included Micro-Pumps, Gas-Mixing Station and ph- and DO control, all integrated into the DASGIP monitoring, documentation and steering system. The DASGIP system was found to provide:
 Reduced working volumes under controlled conditions.
Reduced working volumes under controlled conditions. Similar mixing and oxygen transfer characteristics to stirred systems of a greater volume, resulting in good scalability.
Similar mixing and oxygen transfer characteristics to stirred systems of a greater volume, resulting in good scalability. Suitable results for different mammalian cell culture applications such as process development, cellular engineering and primary cell application.
Suitable results for different mammalian cell culture applications such as process development, cellular engineering and primary cell application.
Fermenter and Bioreactor Process Control
Intelligent Front Modules to measure and control a wide range of parameters like temperature, pH, pO2, pCO2, turbidity, torque, weight, level, etc.
Biologics customized Supervisor Control and Data Acquisition software.
It manages the data acquisition, displays graphs, controls the process, performs quality assurance and enables program processes.
|
|
Fermenter

The Minifor was developed as a result of the need to construct a small laboratory fermenter for volumes from 0.035 to 4.5l.
Based on long personal practical experience in fermentation we wanted to create a fermenter, which was easy to use and with the capacity to measure and control all the important parameters of the biological culture.
The fermenter had to take up minimum space on the bench but with a good access to all parts.
Several fermenters should, when placed side by side be suitable for the optimisation of the parameters of growth of culture or optimisation of biotransformations etc.
Each fermenter should be able to work independently or be connected to a PC for advanced regulation and extensive data treatment.
Heterogenous cell kinetics in tumor
Cell Kinetics
| Cell Kinetics - A Useful Endpoint for Inhalation Toxicology Studies Cells in mitosis are rarely observed in the adult rodent lung. The rate of cell proliferation increases when animals are exposed to agents by inhalation and intratracheal instillation as well as by the blood. Tritiated thymidine and 5-bromo-2'-deoxyuridine (BrdU) incorporation into cells undergoing DNA synthesis are used to assess rates of cell proliferation in the lung. Specifics of the techniques of proper measurement of cell proliferation are important and will be covered in a future paper. It should be noted that few attempts to measure other parameters of the cell cycle such as G1, G2 and M phase have been attempted in the lung. The population of proliferating cells may be epithelial, endothelial, interstitial, PAM (pulmonary alveolar macrophages), or combinations thereof depending upon the exposure agent,and dose and time of exposure. In addition, different areas of the respiratory tract may be affected more than others. For example, the centriacinar (terminal bronchiolar-alveolar) region is most sensitive to ozone. Increases in cell proliferation have generally been considered to be associated with morphological injury as detected with light and electron microscopy. Return to normal levels of proliferation usually corresponds with repair of the lung or cessation of damage. The magnitude of type II cell proliferation in particular correlates with degree of damage to the alveolar region. This correlation was first shown after exposures to oxidant gases such as ozone and NO2. In many instances, cell proliferation increases in the lungs without signs of morphological injury. This phenomenon is often seen when inert or nontoxic particles are inhaled and is related to an influx of inflammatory cells from the blood to the airspaces of the lung. The significance of this transient proliferative response is unclear. It has also been observed after unilateral pneumonectomy , collapse of a lung, and bronchoalveolar lavage. Quite unexpected was the finding that sham exposed control animals in both whole body and nose only inhalation chambers exhibit significant increases in proliferation of all cell types in the lungs. The information obtained from studies of proliferating cells can be useful in determining the mechanisms of action of inhaled agents. The adaption of the lung to ozone as well as oxygen toxicity can be explained with the help of kinetic studies. Much less information is available on cell proliferation during and after exposure to other common air pollutants such as tobacco smoke and diesel exhaust. The paucity of information is probably related to the perceived difficulty in performing cytokinetic studies. Even more helpful in assessing the toxicity of a compound is integration of this information with other changes measured simultaneously in the lungs. These other changes might be in terms of pathology, respiratory mechanics, composition of BAL (bronchoalveolar lavage) fluid, immunology and/or metabolism. These changes will ultimately affect the animal in terms of function. Table 1 is an example of agents known to cause cell proliferation in the lung. Increases in lung cell proliferation may also result in permanent morphological and functional changes in the lungs even after exposure has ceased. Examples include fibrosis, emphysema, hyperplasia, metaplasia, tumor development, and granuloma formation. Unfortuanately cell proliferation studies have not yielded a great deal of information concerning mechanisms of lung tumor development and stem cells of tumors. This may be due to the fact that cancer and precancerous cells do not necessarily have different rates of DNA synthesis compared to normal cells. Other parameters of the cell cycle and cell growth may change such as the growth fraction and rate of cell death. Although it is not impossible to measure these other parameters, it does require a great deal of technical expertise and patience. In summary the adult lung normally shows very little cell proliferation. However, when exposed to various toxic insults, cell proliferation may increase significantly with corresponding morphological signs of injury and repair of the lung. In most cases the lung cells then return to their low steady state rate of cell proliferation. Cell proliferation may be one of the most sensitive indicators of exposure to inhaled agents. |
Enzyme inhibition
Enzyme inhibitors are molecules that reduce or abolish enzyme activity. These are either reversible (i.e., removal of the inhibitor restores enzyme activity) or irreversible (i.e., the inhibitor permanently inactivates the enzyme).
Reversible inhibitors
Reversible enzyme inhibitors can be classified as competitive, uncompetitive, non-competitive or mixed, according to their effects on Km and Vmax. These different effects result from the inhibitor binding to the enzyme E, to the enzyme–substrate complex ES, or to both, as shown in the figure to the right and the table below. The particular type of an inhibitor can be discerned by studying the enzyme kinetics as a function of the inhibitor concentration. The four types of inhibition produce Lineweaver–Burke and Eadie–Hofstee plots[39] that vary in distinctive ways with inhibitor concentration. For brevity, two symbols are used:
![\alpha = 1 + \frac{[\mbox{I}]}{K_{i}}](http://upload.wikimedia.org/math/5/d/0/5d0ef5c67ccd4c852e6cfca33a00b3c1.png) and
and ![\alpha^{\prime} = 1 + \frac{[\mbox{I}]}{K_{i}^{\prime}}](http://upload.wikimedia.org/math/1/3/1/131b809d1c25e2a337f97992bbbc9593.png)
where Ki and K'i are the dissociation constants for binding to the enzyme and to the enzyme–substrate complex, respectively. In the presence of the reversible inhibitor, the enzyme's apparent Km and Vmax become (α/α')Km and (1/α')Vmax, respectively, as shown below for common cases.
| Type of inhibition | Km apparent | Vmax apparent | ||
| Ki only | ( ) ) | competitive | 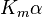 | 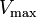 |
| Ki' only | (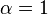 ) ) | uncompetitive | 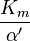 | 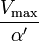 |
| Ki = Ki' | (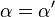 ) ) | non-competitive | 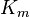 | 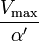 |
| Ki ≠ Ki' | (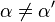 ) ) | mixed |  | 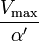 |
Non-linear regression fits of the enzyme kinetics data to the rate equations above[40] can yield accurate estimates of the dissociation constants Ki .
Enzymes Kinetics

Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes, with a focus on their reaction rates. The study of an enzyme's kinetics provides insights into the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled in the cell, and how drugs and poisons can inhibit its activity.
Enzymes are protein molecules that manipulate other molecules — the enzymes' substrates. These target molecules bind to an enzyme's active site and are transformed into products through a series of steps known as the enzymatic mechanism. These mechanisms can be divided into single-substrate and multiple-substrate mechanisms. Kinetic studies on enzymes that only bind one substrate, such as triosephosphate isomerase, aim to measure the affinity with which the enzyme binds this substrate and the turnover rate.
When enzymes bind multiple substrates, such as dihydrofolate reductase (shown right), enzyme kinetics can also show the sequence in which these substrates bind and the sequence in which products are released. An example of enzymes that bind a single substrate and release multiple products are proteases, which cleave one protein substrate into two polypeptide products. Others join two substrates together, such as DNA polymerase linking a nucleotide to DNA. Although these mechanisms are often a complex series of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a conformational change of the enzyme or substrates, such as those involved in the release of product(s) from the enzyme.
Knowledge of the enzyme's structure is helpful in interpreting the kinetic data. For example, the structure can suggest how substrates and products bind during catalysis; what changes occur during the reaction; and even the role of particular amino acid residues in the mechanism. Some enzymes change shape significantly during the mechanism; in such cases, it is helpful to determine the enzyme structure with and without bound substrate analogs that do not undergo the enzymatic reaction.
Not all biological catalysts are protein enzymes; RNA-based catalysts such as ribozymes and ribosomes are essential to many cellular functions, such as RNA splicing and translation. The main difference between ribozymes and enzymes is that the RNA catalysts perform a more limited set of reactions, although their reaction mechanisms and kinetics can be analysed and classified by the same methods.
NASA Tissue cloning bioreactor
NASA has developed a new type of bioreactor that artificially grows tissue in cell cultures. NASA's tissue bioreactor can grow heart tissue, skeletal tissue, ligaments, cancer tissue for study, and other types of tissue.
Bioreactor designs
Bioreactor design is a complex engineering task. Under optimum conditions, the microorganisms or cells are able to perform their desired function with 100 percent rate of success. The bioreactor's environmental conditions like gas (i.e., air, oxygen, nitrogen, carbon dioxide) flow rates, temperature, pH and dissolved oxygen levels, and agitation speed/circulation rate need to be closely monitored and controlled.
Most industrial bioreactor manufacturers use vessels, sensors and a control system networked together.
Fouling can harm the overall sterility and efficiency of the bioreactor, especially the heat exchangers. To avoid it, the bioreactor must be easily cleaned and as smooth as possible (therefore the round shape).
A heat exchanger is needed to maintain the bioprocess at a constant temperature. Biological fermentation is a major source of heat, therefore in most cases bioreactors need refrigeration. They can be refrigerated with an external jacket or, for very large vessels, with internal coils.
In an aerobic process, optimal oxygen transfer is perhaps the most difficult task to accomplish. Oxygen is poorly soluble in water--even less in fermentation broths--and is relatively scarce in air (20.8%). Oxygen transfer is usually helped by agitation, which is also needed to mix nutrients and to keep the fermentation homogeneous. There are, however, limits to the speed of agitation, due both to high power consumption (which is proportional to the cube of the speed of the electric motor) and to the damage to organisms caused by excessive tip speed causing shear stress.
Industrial bioreactors usually employ bacteria or other simple organisms that can withstand the forces of agitation. They are also simple to sustain, requiring only simple nutrient solutions, and can grow at astounding rates.
Sewage Treatment: Bioreactors are also designed to treat sewage and wastewater. In the most efficent of these systems there is a supply of free-flowing, chemically inert media that acts as a receptacle for the bacteria that breaks down the raw sewage. Examples of these bioreactors often have separate, sequential tanks and a mechanical separator or cyclone to speed the division of water and biosolids. In the process, the liquids Biochemical Oxygen Demand BOD is reduced sufficiently to render the contaminated water fit for reuse. The biosolids can be collected for further processing or dried and used as fertilizer.
In bioreactors where the goal is grow cells or tissues for experimental or therapeutic purposes, the design is significantly different from industrial bioreactors. Many cells and tissues, especially mammalian ones, must have a surface or other structural support in order to grow, and agitated environments are often destructive to these cell types and tissues. Higher organisms also need more complex growth medium.
Packed bed
The purpose of a packed bed is typically to improve contact between two phases in a chemical or similar process. Packed beds can be used in a chemical reactor, distillation process, or a scrubber, but packed beds have also been used to store heat in chemical plants. In this case, hot gases are allowed to escape through a vessel that is packed with a refractory material until the packing is hot. Air or other cool gas is then fed back to the plant through the hot bed, thereby pre-heating the gas feed.
Distillation columns with packing are often called packed columns. Columns used in certain types of chromatography consisting of a tube filled with packing material can also be called packed columns and their structure has similarities to packed beds.
The Ergun equation can be used to predict the pressure drop along the length of a packed bed given the fluid velocity, the packing size, and the viscosity and density of the fluid.
The bioreactor

A bioreactor may refer to any device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which is carried out a chemical process which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from liters to cube meters, and are often made of stainless steel.
A bioreactor may also refer to a device or system meant to grow cells or tissues in the context of cell culture. These devices are being developed for use in tissue engineering.
On the basis of mode of operation, a bioreactor may be classified as batch, fed batch or continuous (e.g. Continuous stirred-tank reactor model). An example of a bioreactor is the chemostat.
Organisms growing in bioreactors may be suspended or immobilized . The simplest, where cells are immobilized, is a Petri dish with agar gel. Large scale immobilized cell bioreactors are:
- moving media
- packed bed
- fibrous bed
- membrane
Biochemical Engineering

Biochemical engineering is a branch of chemical engineering or biological engineering that mainly deals with the design and construction of unit processes that involve biological organisms or molecules. Biochemical engineering is often taught as a supplementary option to chemical engineering or biological engineering due to the similarities in both the background subject curriculum and problem-solving techniques used by both professions. Its applications are used in the food, feed, pharmaceutical, biotechnology, and water treatment industries.
